Metallothioneins
Your Body’s Built-In “Metal Mitts”
How to Work With Them
If you’ve ever wondered how the body naturally handles heavy metals, here’s the headline: we come equipped with tiny, cysteine-rich proteins called metallothioneins (MTs) that act like molecular oven mitts—they grab onto metals such as cadmium (Cd), mercury (Hg), lead (Pb), copper (Cu), and zinc (Zn), help buffer their reactivity, and limit damage.
MTs don’t replace chelation; they’re part of your innate detox toolkit.
In this article I’ll explain—in plain English—what MTs do, how to support them, where they fall short, and how I pair a natural chelation program (Dr. Georgiou’s HMD™ protocol) with diet and lifestyle, so metals not only get sequestered but also leave the body.
MTs 101 — What they are and why they matter
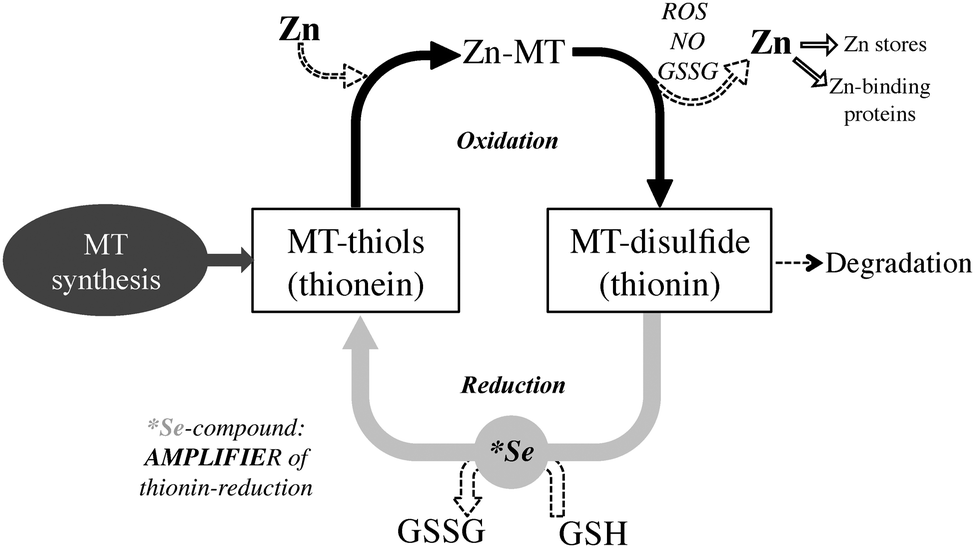
- Small, thiol-rich metal buffers. MTs are very small proteins (~6–7 kDa) packed with sulfur-containing cysteines. Those sulfur groups bind metals tightly; MTs help maintain metal balance (especially zinc/copper) and blunt oxidative stress. Think of them as metal sponges + antioxidants at the cellular level.
- Induced by metals and stress. When cells sense certain metals (Zn, Cd, Cu) or oxidative stress, a transcription factor called MTF-1 flips on MT genes. Zinc is a classic signal that up-regulates MTs—part of the body’s “safety mode” for metal handling.
- Where they work. MT1/MT2 isoforms are abundant in the liver and kidneys (also present in the gut, brain, etc.). In kidneys and liver, they can capture incoming metal ions and reduce their ability to wreak havoc.
How MTs help with specific metals
Cadmium strongly induces MTs. That’s partly protective—MT binding reduces free Cd in tissues—but there’s a catch: Cd–MT complexes are filtered by the kidney and can be reabsorbed by proximal tubule cells (via endocytic pathways such as megalin/cubilin), contributing over time to tubular injury if exposure continues. This is a key reason long-term Cd exposure is tough on kidneys.
Mercury loves sulfur. MTs help bind Hg and can reduce nephrotoxicity; in animal work, zinc pretreatment (which raises MTs) increased Hg binding in the renal cortex and protected against kidney damage. MT–Hg complexes are taken up by proximal tubules; MT overexpression shifts where Hg accumulates and can lessen injury.
MTs bind Pb less avidly than Cd/Hg but still contribute to buffering Pb and other metals while limiting oxidative stress. Across toxicology, MTs are consistently described as protective, inducible metal-binding proteins central to the natural response to heavy metals.
Bottom line: MTs are your first responders—they sequester metals and reduce immediate damage. But sequestration isn’t the same as elimination. For that, we still need the exit ramps (bile → stool, kidneys → urine) working, and often we need binders/chelators so mobilized metals don’t just recirculate.
What MTs can’t do (and why that matters for detox)
- MTs don’t escort metals out of the body by themselves. They hold metals inside cells or in circulating complexes; those complexes must be processed and excreted. Without adequate bile flow, fiber, hydration, mineral balance, and (when appropriate) chelation/binding, metals can re-enter circulation or accumulate in kidneys.
- Chronic exposure can overwhelm the system. With ongoing Cd, for example, you can see rising MT and rising kidney burden over time—protection has limits if exposure isn’t reduced, and elimination isn’t supported.
How to work with metallothioneins (not against them)
1) Provide the raw materials and signals
- Zinc (within safe ranges). Zinc is both a structural partner for MT and a signal (via MTF-1) to up-regulate MT expression. Maintaining zinc sufficiency supports MT-mediated buffering (and gut barrier function). Excess zinc is not better—aim for adequacy through food (seafood, meat, pumpkin seeds) or a modest supplement if deficient.
- Protein & sulfur amino acids. MTs are cysteine-rich; adequate protein (and sulfur amino acids from eggs, legumes, fish, meat) supports thiol-based defenses.
2) Reduce incoming load
- Water: use NSF/ANSI 53 (or RO 58) certified filtration for metals, fix sources.
- Food: choose low-mercury fish, handle rice smartly (rinse; extra-water cooking) for arsenic.
(General toxicology guidance; not all cited here to keep focus on MTs.)
3) Add exit support when you mobilize
- Fiber (25–35 g/day) to bind bile-carried metals in the gut.
- Hydration + electrolytes to support renal excretion.
- Binders/chelators to capture mobilized metals so they don’t rebound.
Where Dr. Georgiou’s HMD™ protocol fits—and how it complements MTs
When I want a gentle, programmatic approach for chronic, low-to-moderate body burdens, I often use HMD™ alongside the MT-supportive steps above.
What it is:
- HMD™ liquid (mobilizer): a blend centered on Coriandrum sativum (cilantro), Chlorella Growth Factor, and a homaccord of Chlorella pyrenoidosa.
- HMD™ LAVAGE (drainage): herbs supporting liver, kidneys, lymph—the “exit ramps.”
- HMD™ Organic Chlorella (binder): binds metals in the gut to interrupt enterohepatic recirculation.
Why I give it oxygen, in a science-based plan:
Unlike most natural blends, HMD™ is one of the few to report randomized, double-blind, placebo-controlled human trials—conducted in ~350 metal foundry workers over several years—to screen multiple natural candidates and converge on the final HMD™ combo that increased urinary/fecal metal excretion under provocation. (These are in complementary/alternative outlets; I’m transparent about that so you can weigh it.)
How HMD™ and MTs play together:
- MTs quickly bind metals mobilized from tissues—great for safety, but retention can rise unless you create a clear exit.
- HMD™ provides mobilization (cilantro/CGF) plus a gut binder (chlorella) and drainage—so as MT and glutathione shuttle metals toward bile, there’s something waiting in the gut to capture them and carry them out.
- Chlorella also has preclinical data showing it can reduce cadmium accumulation and, when given with exposure, limit absorption—consistent with its role as a binder rather than an intracellular chelator. (After a load is already stored, chlorella alone doesn’t do much—hence the value of the combined protocol.)
Adult guidance (from the program):
- HMD™: 45 drops, 3×/day, 10–15 min before meals (titrate up if sensitive).
- LAVAGE: 25 drops, 3×/day (often in the same glass).
- Organic Chlorella: 2 caps (~600 mg), 2×/day with meals.
Typical cycle: 60–90 days, with symptom tracking and, when appropriate, urinary metals (creatinine-normalized).
Important safety note: Pregnancy/breastfeeding, kidney/liver disease, or high acute exposures require clinician oversight. In severe poisoning, guideline-driven medical chelation takes priority.
FAQs I hear from readers
Does zinc “boost detox” by itself?
Zinc mainly supports MT expression and gut barrier health; that helps buffer metals and limit absorption. It’s a cofactor, not a chelator. Over-supplementing can unbalance other minerals—aim for adequacy.
Can MTs trap mercury in kidneys? Isn’t that bad?
It’s nuanced. More MT often means less toxicity, but MT–Hg complexes can be taken up by proximal tubule cells. That’s exactly why I couple mobilization with binders + drainage and keep exposures low—so captured metals exit rather than recirculate.
Is there proof that sweating helps?
Separate from MTs, induced sweating can carry arsenic, cadmium, lead, and mercury—plus some organics like BPA—in small human studies. I use sweat sessions as an adjunct with hydration/electrolytes and gut binders. (Different article; quick references available on DetoxMetals.)
A simple, MT-friendly, HMD-supported plan
- Reduce inputs (filters, low-mercury seafood, dust control).
- Feed the system (adequate protein, zinc sufficiency, colorful plants).
- Run HMD™ in 90-day blocks (mobilize → bind → drain), with fiber (25–35 g/day), hydration, and regular bowels to keep metals moving out.
- Optional adjuncts (as tolerated): light exercise or gentle heat/sweat with electrolytes.
- Personalize and monitor: energy, sleep, skin, headaches, bowels; consider spot urinary metals with your clinician.
My take
Metallothioneins are why many people tolerate daily trace exposures without obvious symptoms—they bind and buffer metals before they can do their worst. But buffering is not removal.
If you want a practical, low-drama path to lower body burden, combine: (1) source control and MT support (zinc sufficiency, protein), with (2) a mobilize–bind–drain program such as HMD™—so the metals MTs catch today are the metals you excrete tomorrow.
Educational only; not medical advice. If you have kidney/liver disease, are pregnant/breastfeeding, or suspect high acute exposure, work with a qualified clinician—standard medical chelation may be indicated depending on the case.