Epigenetic Effects of Early-Life Heavy-Metal Exposure
What it means for babies and kids, and how I think about prevention and safe detox
Including Dr. Georgiou’s HMD™ protocol
When I talk with parents about heavy metals, I don’t start with scary lists of symptoms. I start with epigenetics—the body’s way of using “dimmer switches” on genes. Those switches help turn genes up or down without changing the DNA code itself.
Early in life (from pre-conception through pregnancy and the first years), those switches are incredibly sensitive. That’s why heavy-metal exposure in early life can leave lasting fingerprints on health—sometimes years after the exposure—and why a smart plan focuses on reducing exposure, supporting biology, and safely eliminating metals at any age.
Below, I’ll explain epigenetics in plain English, walk through what the science shows about arsenic, mercury, lead, and cadmium in pregnancy/infancy, and finish with a practical plan—including where Dr. Georgiou’s HMD™ protocol fits as a gentle option to help the body off-load metals.
Epigenetics, in simple terms
Think of your DNA as the hardware and epigenetics as the software that tells the hardware what to do. The main “software tools” are:
- DNA methylation – tiny chemical tags (methyl groups) attached to DNA that act like dimmer switches on genes.
- Histone modifications – changes to the proteins that package DNA, which can loosen or tighten access to genes.
- Non-coding RNAs – small RNA messages that fine-tune gene activity.
These mechanisms can turn genes “on” or “off” without changing the DNA letters. They also respond to environment—nutrition, stress, and yes, chemical exposures—especially early in life. The good news: many epigenetic marks are dynamic, which is why lifestyle and exposure reduction still matter later on.
Why early-life windows matter so much
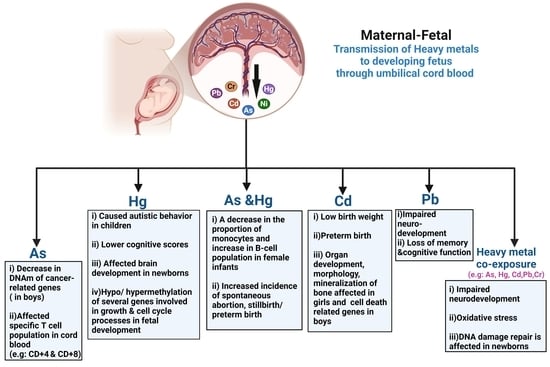
During pregnancy and early childhood, tissues are being built at high speed. The fetus relies on the mother’s nutrients—and is also exposed to whatever crosses the placenta (including some metals). Studies of cord blood and placenta repeatedly show that in-utero metal exposure tracks with changes in newborn DNA methylation, often in genes tied to development and the brain. Some of those changes persist into childhood, which is why they may influence later health.
What the science shows—metal by metal
Arsenic (As)
Arsenic in drinking water and food (e.g., some rice) is a classic epigenetic toxicant. Multiple cohorts report that prenatal arsenic exposure is associated with altered global and gene-specific DNA methylation in cord blood, sometimes differently in boys vs girls. Some epigenetic changes even help explain (mediate) arsenic’s effects on birth outcomes such as gestational age. Emerging work continues to connect prenatal arsenic, DNA methylation differences, and neurodevelopment in toddlers.
What this implies: clean water and smart food choices during pregnancy (plus after) are non-negotiable; epigenetic data back that up.
Mercury (Hg)
Most prenatal mercury exposure comes from methylmercury in certain fish. Cord-blood studies in Project Viva and other cohorts found that higher prenatal mercury correlates with genome-wide DNA methylation shifts at birth; in some datasets, methylation changes persisted into mid-childhood.
One study also found lower 5-hydroxymethylcytosine (5hmC)—a mark involved in active DNA demethylation—in babies with higher prenatal mercury, suggesting mercury can alter the balance of epigenetic marks that keep development on track.
What this implies: choose low-mercury fish during pregnancy and childhood; if past exposure was likely, a gentle elimination plan later can help reduce ongoing epigenetic “pressure.”
Lead (Pb)
Lead exposure in pregnancy and early childhood is still far too common. Beyond the well-known IQ effects, epigenome-wide studies show that prenatal lead is linked with cord-blood DNA methylation differences, and even dried blood spots from lead-exposed infants show sex-specific methylation changes. These patterns often appear in genes related to neurodevelopment—right where you’d expect lead to matter most.
What this implies: test and filter water, remediate old paint/dust, and screen kids at risk. Even if exposure happened years ago, lowering body burden may still be valuable.
Cadmium (Cd)
Cadmium (from cigarette smoke, some foods, and industrial sources) is another epigenetic actor. Placental and cord-blood studies report consistent DNA methylation differences with maternal cadmium; some changes track with growth measures at birth. Several groups (including NEST and INMA) have shown distinct methylation “footprints” in newborns of cadmium-exposed mothers, and even imprinted regions like MEG3 can be affected. Some signals appear to persist, though the duration varies by study.
What this implies: avoid tobacco smoke; focus on a mineral-replete diet (zinc/iron status can modify cadmium uptake) and consider long-horizon detox if body burden is elevated.
Mechanisms: how metals tweak the “dimmer switches”
Heavy metals don’t randomly flip epigenetic switches. They often act through:
- Oxidative stress and redox shifts that influence enzymes (DNMTs, TETs) writing/erasing methyl marks.
- One-carbon metabolism demands (folate, B12, choline, betaine) that supply methyl groups.
- Direct effects on proteins (e.g., zinc finger transcription factors).
Several reviews tie oxidative stress tightly to epigenetic remodeling, while others lay out how epigenetic data can improve metal risk assessment across arsenic, cadmium, chromium, lead, and mercury.
How long do these marks last—and can we change them?
This is the hopeful part. Some epigenetic differences persist into early or mid-childhood after prenatal metal exposure, but epigenetics is not fate. It’s responsive to environment across life. Public-health sources (CDC, NIH) emphasize that many epigenetic marks are potentially reversible—which is why exposure reduction, nutrition, movement, sleep, and stress care still matter, even years later.
A note on “transgenerational” effects: animal studies show that prenatal metal exposures (especially cadmium and arsenic) can leave multigenerational epigenetic signatures; human evidence is emerging but not definitive yet. As methods improve, we’re learning how sperm/egg epigenetics may transmit some “memory” of exposure across generations.
Practical steps I recommend (before we talk detox)
- Water first.
- Test your water where possible. Use certified filters for lead/arsenic (look for NSF/ANSI 53 or 58 for RO systems). This single step protects pregnancy and children as much as almost anything else. (Your local water reports and NSF listings can guide model choice.)
- Fish the smart way.
- Keep seafood (Omega-3s!) but choose low-mercury options like wild salmon, sardines, smelt, trout. Limit large predatory fish during pregnancy and early childhood.
- Household hygiene.
- Wet-mop, HEPA vacuum, and wash hands before meals—especially in older homes where lead dust is a risk.
- Nutrition for methylation & antioxidants.
- Folate-rich greens/beans, B12, choline (eggs, legumes), betaine (beets), selenium (Brazil nuts), zinc (pumpkin seeds, seafood). These don’t “erase” marks, but they support the enzymes that maintain a healthy epigenome—and help buffer oxidative stress triggered by metals.
- Microbiome basics.
- Fiber (25–35 g/day), fermented foods, and regular bowel movements help interrupt re-circulation of metals excreted in bile, so more leaves in stool instead of bouncing back.
Where safe elimination fits—at any age
It’s one thing to avoid new exposure; it’s another to lower body burden after the fact. That’s where detox (properly done) can support the biology that epigenetics is tracking.
- Chelation and natural binders don’t change epigenetic marks directly. What they do is reduce ongoing metal stress, which can let those epigenetic dimmers drift back toward healthier settings over time—especially alongside good nutrition, sleep, movement, and stress care.
- For acute, high-level poisoning, conventional chelators (e.g., DMSA, CaNa₂EDTA) under medical supervision are the standard; for chronic, low-to-moderate body burdens where people want gentler progress, I often start with a natural mobilize-bind-drain approach.
Dr. Georgiou’s HMD™ protocol—why I use it as a gentle, structured option
HMD™ (Heavy Metal Detox) is a three-part program developed and tested over several years in metal foundry workers:
- HMD™ liquid (mobilizer): a blend built around Coriandrum sativum (cilantro), Chlorella Growth Factor, and a homaccord of Chlorella pyrenoidosa.
- HMD™ LAVAGE (drainage): herbal support for liver, kidneys, lymph, i.e., the “exit ramps.”
- HMD™ Organic Chlorella (binder): catches metals dumped in bile so they exit in stool instead of being re-absorbed.
What makes HMD™ notable: it’s one of the very few natural protocols that has been tested in randomized, double-blind, placebo-controlled trials—reportedly with ~350 foundry workers over multiple years. Those trials compared many natural candidates, then converged on the HMD™ combo that increased urinary and fecal excretion across multiple metals during provocation testing.
Adult dosing (from the program’s guidance):
- HMD™: 45 drops, 3×/day, 10–15 minutes before meals (I titrate up slowly for sensitive people).
- LAVAGE: 25 drops, 3×/day (often in the same glass as HMD™).
- Organic Chlorella: 2 capsules (~600 mg), 2×/day with breakfast and dinner.
Children 2+ can use weight-based doses with practitioner guidance. Typical course: 60–90 days, then reassess symptoms and (ideally) labs.
Why this fits an epigenetic lens: the goal is to lower the body’s metal pressure steadily, with minimal redistribution symptoms. I keep the foundations in place (fiber, hydration, minerals, bile/microbiome support), and I adjust dose/spacing based on energy, sleep, skin, headaches, and bowel regularity. That combination tends to be sustainable, which matters more than heroics.
Safety note: If you’re pregnant or breastfeeding, have kidney/liver disease, or take interacting meds, work with a qualified clinician for any detox (natural or conventional). In known or suspected high-level poisoning, standard medical chelators under supervision are the right tool.
A quick FAQ
Can detox “fix” epigenetic marks?
Not directly. But by removing metals, you reduce the ongoing triggers that maintain unhealthy marks. Paired with nutrition, sleep, movement, and stress care, the epigenome can shift in a healthier direction.
Is early-life exposure really linked to later outcomes?
Yes—multiple cohorts show cord-blood or placental methylation changes with prenatal arsenic, mercury, lead, or cadmium; some changes persist into childhood and cluster in neurodevelopmental pathways. That’s the core of the “developmental origins” idea.
What about “intergenerational” effects?
Animals show convincing multigenerational epigenetic signals after metal exposures; in humans the evidence is emerging, but we need more longitudinal work. Meanwhile, it’s wise to minimize exposure before conception and during pregnancy.
My bottom line
- Epigenetics is how experience talks to genes. In pregnancy and early childhood, that conversation is loud—and metals are powerful speakers.
- The evidence is strong that early-life exposure to arsenic, mercury, lead, and cadmium can shift newborn (and sometimes childhood) DNA methylation, especially in brain- and growth-related pathways.
- You can act on two fronts: (1) prevention (water, food, household hygiene, nutrition) and (2) safe elimination of existing body burden.
- For the second front, Dr. Georgiou’s HMD™ protocol gives a gentle, structured way to help remove metals at any age, backed by double-blind human testing that’s rare among natural options—alongside the foundations that support a resilient epigenome.
Educational only; not medical advice. If you’re pregnant/breastfeeding, have kidney or liver disease, or take interacting medicines, work with a qualified clinician before starting any detox program.